electron configuration for rb|electron configuration for nitrogen : Tagatay The ground state electron configuration of rubidium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1. This electron configuration shows that the last shell of rubidium has only an electron. Therefore, the valence electrons of rubidiumare one. The elements that have 1, 2, or 3 electrons in the last shell donate the . Tingnan ang higit pa Premier League Darts Tips for Night 11 Luke Humphries -1.5 Handicap vs Peter Wright & Luke Littler to Beat Rob Cross @ 1/1 Ladbrokes. Luke Humphries has been below par by his own high standards in recent weeks but I’m prepared to back him to overcome a -1.5 handicap against Peter Wright as the first half of an even-money double .
PH0 · noble gas configuration of rb
PH1 · how to write electron configuration
PH2 · electron configuration for nitrogen
PH3 · electron configuration for every element
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · co3+ ground state
PH7 · abbreviated electron configuration list
PH8 · Iba pa
About Potato Corner. In October 1992, Potato Corner launched its first location in the Philippines and started franchising the following year. Today, it has evolved from modest carts into in-line stores that can be found practically anywhere across the world, including malls, hospitals, schools, bus stops, and amusement parks.Today’s MPBL schedule features some of the league’s top teams, and we’ll be taking a closer look at the top 5 teams with the best odds for today’s schedule. First on the list is the San Juan Knights. The .
electron configuration for rb*******The ground state electron configuration of rubidium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1. This electron configuration shows that the last shell of rubidium has only an electron. Therefore, the valence electrons of rubidiumare one. The elements that have 1, 2, or 3 electrons in the last shell donate the . Tingnan ang higit pa
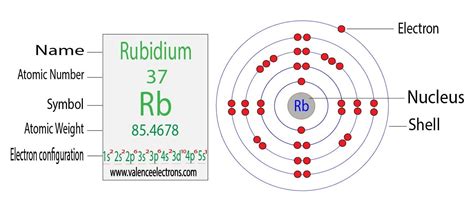
The total number of electrons in rubidium is thirty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in rubidium in specific rules in different . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof the atom revolve around the nucleus in . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Mar 23, 2023 Rb+ Electron Configuration (Rubidium Ion) In this video we will write the electron configuration for Rb+, the Rubidium ion. We’ll also look at why Rubidium forms a 1+ ion and how the. Wayne Breslyn. 738K subscribers. Subscribed. 465. 56K views 4 years ago. In order to write the Rb electron configuration we first need to know the number of electrons for the Rb atom.

The electron configuration of Rubidium simply implies making the distribution of Rubidium’s electron in the molecular orbital. Rubidium is considered to be the highly reactive element and it reacts .Electron configuration of Rubidium. The electron configuration of Rubidium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1. Rubidium is the chemical element whose atomic number .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .Electron configuration for rubidium. The history of Rubidium. Periodic table history. Identifiers. List of unique identifiers for Rubidium in various chemical registry databases. .
electron configuration for rb The group number that an element is in determines the number of its valence electrons. Periodic table of elements (wikipedia.org) For example, chlorine is in group 7, so it has seven valence electrons. . Today in this video, we will help you determine the electron configuration for the Rubidium element. It is a group 1 and period five element. For determining.Element 37 of Periodic table is Rubidium with atomic number 37, atomic weight 85.4678. Rubidium, symbol Rb, has a Body Centered Cubic structure and Silver color. Rubidium is a Alkali Metal element. It is part of group 1 (lithium family). Know everything about Rubidium Facts, Physical Properties, Chemical Properties, Electronic configuration . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully . Rubidium -. Rb: properties of free atoms. Rubidium atoms have 37 electrons and the shell structure is 2.8.18.8.1. The ground state electron configuration of ground state gaseous neutral rubidium is [ Kr .
Rubidium is a chemical element with atomic number 37 which means there are 37 protons and 37 electrons in the atomic structure.The chemical symbol for Rubidium is Rb. Electron Configuration and Oxidation States of Rubidium. Electron configuration of Rubidium is [Kr] 5s1. Possible oxidation states are +1. Electron ConfigurationUsually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance). According to the rule of electron configuration, a substance first fills s subshell, then it fills p subshell, then it fills d subshell. The maximum number of electrons that can be filled in the s subshell is two.electron configuration for rb electron configuration for nitrogenThe same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . Rubidium (Rb) has a +1 ion, will have the same electron configuration as krypton (Kr) because the +1 status means it has lost an electron. The configuration is written 1s22s22p63s23p64s23d104p6.
electron configuration for nitrogen The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .
For example, take the elements in the first column of the periodic table: H, Li, Na, K, Rb, and Cs. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted: Electrons, electron configurations, and the valence shell electron configuration highlighted. H: 1s 1; Li: The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Rubidium is a chemical element of the periodic table with chemical symbol Rb and atomic number 37 with an atomic weight of 85.4678 u and is classed as a alkali metal. . Electron configuration for rubidium. Electron configuration. Shorthand configuration [Kr] 5s 1: Electron configuration. Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 .
Rb I Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 0 4s 2 4p 6 5s 2 S 1 / 2 Ionization energy 33690.81 cm-1 (4.177128 eV) Ref. J61b Rb II Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 0 4s 2 4p 6 1 S 0 Ionization energy 220105.0 cm-1 (27.2895 eV) Ref. R75-1 .
In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom (there are 38 electrons). When we write the c. Today in this video, we will help you determine the electron configuration for the Rubidium element. It is a group 1 and period five element. For determining.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).For example, take the elements in the first column of the periodic table: H, Li, Na, K, Rb, and Cs. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted: Electrons, electron configurations, and the valence shell electron configuration highlighted. H: 1s 1; Li:Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .The atomic number of rubidium is 37, which means it has 37 electrons. Now it is possible to find the orbital notation of rubidium very easily through electron configuration. That is, the orbital notation of rubidium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1.
Pedro De la Vega (born 7 February 2001) is an Argentine footballer who plays as a right winger for Argentine club Lanús. In the game FIFA 21, his overall rating is 74.
electron configuration for rb|electron configuration for nitrogen